Cross-Laboratory Experimental Study of Non-Noble-Metal Electrocatalysts for the Oxygen Reduction Rea
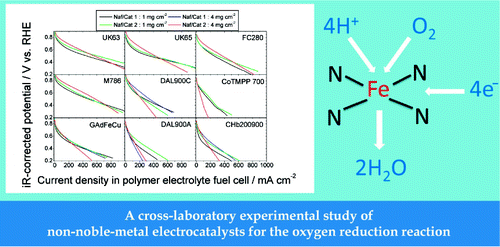
Nine non-noble-metal catalysts (NNMCs) from five different laboratories were investigated for the catalysis of O2 electroreduction in an acidic medium. The catalyst precursors were synthesized by wet impregnation, planetary ball milling, a foaming-agent technique, or a templating method. All catalyst precursors were subjected to one or more heat treatments at 700−1050 °C in an inert or reactive atmosphere. These catalysts underwent an identical set of electrochemical characterizations, including rotating-disk-electrode and polymer−electrolyte membrane fuel cell (PEMFC) tests and voltammetry under N2. Ex situ characterization was comprised of X-ray photoelectron spectroscopy, neutron activation analysis, scanning electron microscopy, and N2 adsorption and its analysis with an advanced model for carbonaceous powders. In PEMFC, several NNMCs display mass activities of 10−20 A g−1 at 0.8 V versus a reversible hydrogen electrode, and one shows 80 A g−1. The latter value corresponds to a volumetric activity of 19 A cm−3 under reference conditions and represents one-seventh of the target defined by the U.S. Department of Energy for 2010 (130 A cm−3). The activity of all NNMCs is mainly governed by the microporous surface area, and active sites seem to be hosted in pore sizes of 5−15 Å. The nitrogen and metal (iron or cobalt) seem to be present in sufficient amounts in the NNMCs and do not limit activity. The paper discusses probable directions for synthesizing more active NNMCs. This could be achieved through multiple pyrolysis steps, ball-milling steps, and control of the powder morphology by the addition of foaming agents and/or sulfur.